UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 6-K
REPORT OF FOREIGN ISSUER
PURSUANT TO RULE 13a-16 OR 15d-16
OF THE SECURITIES EXCHANGE ACT OF 1934
For the month of February 2023
(Commission File No. 001-40241)
LAVA Therapeutics N.V.
(Translation of registrant’s name into English)
Yalelaan 60
3584 CM Utrecht, The Netherlands
(Address of principal executive offices)
Indicate by check mark whether the registrant files or will file annual reports under cover Form 20-F or Form 40-F.
Form 20-F ☒ Form 40-F ☐
Indicate by check mark if the registrant is submitting the Form 6-K in paper as permitted by Regulation S-T Rule 101 (b) (1):
Yes ☐ No ☐
Indicate by check mark if the registrant is submitting the Form 6-K in paper as permitted by Regulation S-T Rule 101 (b) (7):
Yes ☐ No ☐
LAVA Therapeutics, N.V.
On February 16, 2023, LAVA Therapeutics, N.V. (Company) issued a press release announcing initial data from the ongoing Phase 1/2a Clinical Trial of LAVA-1207 in refractory metastatic castration-resistant prostate cancer (mCRPC) in a poster presentation at the American Society of Clinical Oncology (ASCO) Genitourinary Cancers Symposium. A copy of this press release is filed herewith as Exhibit 99.1.
On February 16, 2023, the Company also updated its Investor Presentation on the Company’s website. A copy of the investor presentation is filed herewith as Exhibit 99.2.
EXHIBIT LIST
Exhibit |
| Description |
99.1 | | |
99.2 | |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereto duly authorized.
| LAVA Therapeutics, N.V. | |
| (Registrant) | |
| | |
Date: February 16, 2023 | By: | /s/ Fred Powell |
| Fred Powell | |
| Chief Financial Officer | |

Exhibit 99.1
LAVA Therapeutics Announces Initial Data from the Ongoing Phase 1/2a Clinical Trial of LAVA-1207 in Therapy Refractory mCRPC at the 2023 ASCO GU Symposium
| ● | Favorable safety profile to date, with no occurrence of high-grade (>2) cytokine release syndrome or dose-limiting toxicities |
| ● | Preliminary signs of anti-tumor activity were observed, with iRECIST stable disease (iSD) in 8 out of 14 evaluable patients at week 8 and PSA levels stabilizing or decreasing in heavily pre-treated patients |
| ● | Dose escalation is ongoing |
Utrecht, The Netherlands and Philadelphia, Pa., USA – February 16, 2023 – LAVA Therapeutics N.V. (Nasdaq: LVTX), a clinical stage immuno-oncology company focused on developing its proprietary Gammabody™ platform of bispecific gamma-delta T cell engagers to transform the treatment of cancer, today announced initial clinical data from its ongoing Phase 1/2a study of LAVA-1207 in patients with therapy refractory metastatic castration resistant prostate cancer (mCRPC). The data are presented in a poster presentation at the American Society of Clinical Oncology Genitourinary Cancers Symposium (ASCO GU) taking place in San Francisco from February 16-18, 2023.
“These early data from the first five cohorts of our Phase 1/2a study indicate LAVA-1207 to have a favorable safety profile in patients with therapy refractory metastatic castration resistant prostate cancer. Importantly, preliminary signs of clinical activity were observed with disease stabilization and PSA reduction during dose escalation in these heavily pretreated patients,” said Niven Mehra, M.D., Ph.D., medical oncologist at the Radboud University Medical Center in Nijmegen, The Netherlands. “We are encouraged by the progress of this trial and will continue to enroll patients for additional cohorts.”
LAVA-1207 is an Fc-containing humanized bispecific antibody that directly engages prostate-specific membrane antigen (PSMA) and the Vδ2-T cell receptor chain of Vγ9Vδ2-T cells to mediate potent killing of PSMA-expressing prostate cancer cells. The objectives of the Phase 1/2a study (EudraCT 2021-001789-39; NCT05369000) are to investigate safety and tolerability, evaluate pharmacokinetic and pharmacodynamic effects, immunogenicity and preliminary antitumor activity of LAVA-1207. LAVA-1207 is administered via intravenous infusion every two weeks.
The data presented to date show that a total of 20 patients have been treated with doses ranging from 1.5 to 120 micrograms of LAVA-1207, with treatment duration ranging from 4 to 38 weeks. The safety profile is favorable to date, without occurrence of high grade (>2) cytokine release syndrome or dose-limiting toxicities. LAVA-1207 showed predictable and linear pharmacokinetics and on-mechanism pharmacodynamics including Vγ9Vδ2-T cell activation. Preliminary signs of anti-tumor activity were observed at week 8, with iRECIST stable disease (iSD) in 8 out of 14 evaluable patients and PSA levels stabilizing or decreasing. The largest overall decrease in PSA was 61% (46% vs baseline). The patient improved clinically with improvement in pain and fatigue. Dose escalation is continuing both in Europe and the U.S.

“We are encouraged by these initial data for LAVA-1207,” said Stephen Hurly, president and chief executive officer of LAVA Therapeutics. “At LAVA Therapeutics, we are committed to transforming cancer therapy. I am thrilled to see our second clinical asset continuing to move forward, and an emerging safety profile with the potential for differentiation from prior generation PSMA directed bispecific T-cell engagers.”
Details of the poster presentation are as follows:
Abstract #: 153
Abstract Title: Early dose escalation of LAVA-1207, a novel bispecific gamma-delta T cell engager (Gammabody™), in metastatic castration-resistant prostate cancer (mCRPC) patients
Session Title: Poster Session A: Prostate Cancer
Poster Board #: E13
Session Date: Thursday, February 16, 2023
Session Time: 11:30 AM-1:00 PM PT; 5:45 PM-6:45 PM PT
Presenter: Niven Mehra, MD, PhD, Department of Medical Oncology, Radboud University Medical Center, Nijmegen, The Netherlands
LAVA-1207
LAVA-1207 is a Gammabody™ that conditionally activates Vγ9Vδ2 (Vgamma9 Vdelta2) T cells upon crosslinking to prostate-specific membrane antigen (PSMA) to trigger the potent and preferential killing of PSMA-positive tumor cells, including metastatic castration-resistant prostate cancer (mCRPC).
About LAVA Therapeutics
LAVA Therapeutics N.V. is a clinical-stage immuno-oncology company utilizing its proprietary Gammabody™ platform to develop a portfolio of bispecific gamma-delta T cell engagers for the potential treatment of solid and hematologic malignancies. The Company utilizes bispecific antibodies engineered to selectively kill cancer cells by triggering Vγ9Vδ2 (Vgamma9 Vdelta2) T cell antitumor effector functions upon cross-linking to tumor-associated antigens. LAVA-051, the Company’s lead candidate for the treatment of multiple myeloma, chronic lymphocytic leukemia, and acute myeloid leukemia, is enrolling patients in a Phase 1/2a clinical study (EudraCT 2020-004583-26; NCT04887259). A Phase 1/2a clinical study to evaluate LAVA-1207 in patients with metastatic castration-resistant prostate cancer (mCRPC) is also enrolling (EudraCT 2021-001789-39; NCT05369000). For more information, please visit www.lavatherapeutics.com, and follow us on LinkedIn, Twitter and YouTube.
LAVA’s Cautionary Note on Forward-Looking Statements
This press release contains forward-looking statements, including in respect to the company’s anticipated growth and clinical developments plans, and the timing and results of clinical trials. Words such as “anticipate,” “believe,” “could,” “will,” “may,” “expect,” “should,” “plan,” “intend,” “estimate,” “potential” and similar expressions (as well as other words or expressions referencing future events,

conditions or circumstances) are intended to identify forward-looking statements. These forward-looking statements are based on LAVA’s expectations and assumptions as of the date of this press release and are subject to various risks and uncertainties that may cause actual results to differ materially from these forward-looking statements. Forward-looking statements contained in this press release include, but are not limited to, statements about the preclinical & clinical data, clinical development and scope of clinical trials, and the potential use of our product candidates to treat various tumor targets. Many factors, risks and uncertainties may cause differences between current expectations and actual results including, among other things, the timing and results of our research and development programs and preclinical and clinical trials, our ability to obtain regulatory approval for and commercialize our product candidates, our ability to leverage our initial programs to develop additional product candidates using our GammabodyTM platform, and the failure of LAVA’s collaborators to support or advance collaborations or our product candidates. The COVID-19 pandemic may disrupt our business and that of the third parties on which we depend, including delaying or otherwise disrupting our clinical trials and preclinical studies, manufacturing and supply chain, or impairing employee productivity. In addition, there may be adverse effects on our business condition and results from general economic and market conditions and overall fluctuations in the United States and international equity markets, including deteriorating market conditions due to investor concerns regarding inflation and hostilities between Russia and Ukraine. LAVA assumes no obligation to update any forward-looking statements contained herein to reflect any change in expectations, even as new information becomes available.
CONTACTS
Argot Partners (IR/Media)
212-600-1902
lava@argotpartners.com
| Gamma delta T cell engagers for the development of next-generation cancer therapeutics Corporate Presentation February 2023 |
| Legal Disclosure: Forward-looking Statements 2 This presentation contains statements that constitute forward-looking statements. Many of the forward-looking statements contained in this presentation can be identified by the use of forward-looking words such as “anticipate,” “believe,” “could,” “expect,” “should,” “plan,” “intend,” “estimate” and “potential,” and similar terms and phrases. Forward-looking statements appear in a number of places in this presentation and include, but are not limited to, statements regarding our intent, belief or current expectations. Forward-looking statements are based on our management’s beliefs and assumptions and on information currently available to our management. Such statements are subject to risks and uncertainties, and actual results may differ materially from those expressed or implied in the forward-looking statements due to various important factors. These risk and uncertainties include, among other things, the timing and results of our research and development programs, preclinical studies and clinical trials, including the timing of our clinical trials for LAVA-051 and LAVA-1207, and the submission of INDs or CTAs for our other product candidates; our ability to develop and obtain regulatory approval for and commercialize any of our product candidates; the failure of LAVA’s collaborators to support or advance collaborations or our product candidates; our ability to leverage our initial programs to develop additional product candidates using our Gammabody™ platform; and the risk that positive results in a preclinical study or clinical trial may not be replicated in subsequent trials or success in early stage clinical trials may not be predictive of results in later stage clinical trials. For a discussion of other risks and uncertainties, and other important factors, any of which could cause our actual results to differ from those contained in the forward‐looking statements, see the "Risk Factors" section, as well as discussions of potential risks, uncertainties and other important factors, in the filings we make with the Securities and Exchange Commission from time to time. Because forward-looking statements are inherently subject to risks and uncertainties, some of which cannot be predicted or quantified and some of which are beyond our control, you should not rely on these forward-looking statements as predictions of future events. Although we believe that we have a reasonable basis for each forward-looking statement contained in this presentation, the events and circumstances reflected in our forward-looking statements may not be achieved or occur and actual results could differ materially from those projected in the forward-looking statements. We qualify all of our forward-looking statements by these cautionary statements. Any forward-looking statements represent the Company’s views only as of the date of this presentation and do not represent its views as of any subsequent date. The Company explicitly disclaims any obligation to update any forward-looking statements. By attending this presentation, you acknowledge and agree that you are cautioned not to place undue reliance on any forward-looking statements, and that you will conduct your own analysis and be solely responsible for forming your own view of the potential future performance of the Company. |
| Pioneering Next-Generation Cancer Therapeutics ©LAVA Therapeutics 2023 3 • Bispecific antibody platform to engage Vγ9Vδ2 T cells for highly specific tumor cell killing – Leverage the unique quality of Vγ9Vδ2 T cells to selectively kill tumor cells while sparing normal cells – Fully modular approach amenable to the use of existing and newly generated antibodies from any platform – Gammabody™ combines potent tumor cell killing with no activation of suppressor T cells, low potential for on-target/off-tumor toxicity, and cytokine release syndrome Proprietary Gammabody™ platform • 2 programs in Phase 1/2a trials – LAVA-051 (CD1d), initial data released ASCO and ASH 2022. Additional data expected to be released 2023 – LAVA-1207 (PSMA), initial data presented at ASCO-GU (Q1 2023). Additional data expected to be released H2 2023 Clinical stage company • LAVA-1266 (CD123) projected to enter the clinic in the next 2 years and LAVA-1223 (EGFR, licensed to Seagen) • Multiple additional preclinical programs – Includes partnered discovery program with Janssen (J&J) Robust pipeline • $142.7M (Q3 2022) in cash and investments; >24 months cash runway • Collaborations with Janssen (J&J) and Seagen Solid financials and partnerships |
| MM: multiple myeloma CLL: chronic lymphocytic leukemia AML: acute myeloid leukemia PSMA: prostate-specific membrane antigen EGFR: epidermal growth factor receptor mCRPC: metastatic castration-resistant prostate cancer Gammabody™ Pipeline: Potential in Hematological and Solid Tumor Indications 4 Candidate Target Indication(s) Discovery Preclinical Phase 1 Phase 2 Milestones LAVA-051 CD1d MM CLL AML • Initial data released ASCO 2022 • Additional data in 2023 LAVA-1207 PSMA mCRPC • Initial data released ASCO GU 2023 • Additional data 2H 2023 LAVA-1223 EGFR Solid Tumors • Licensed to Seagen Sep 2022 LAVA-1266 CD123 Hematologic Malignancies • IND/CTA filing expected in 2024 LAVA-1278 CD40 Hematologic Malignancies Janssen Collaboration undisclosed Hematologic malignancy Solid Tumor ©LAVA Therapeutics 2023 |
| Ton Adang, PhD CDO • Vast experience in drug development • Extensive experience in product discovery and project management (e.g., KEYTRUDA) HansvanderVliet,MD,PhD CSO • Inventor of LAVA’s gamma delta T cell engager platform • Medical oncologist, extensive experience in pre-clinical and clinical research Team Led by Experienced Leaders in the Biotech and Pharma Field Paul Parren, PhD EVP • Industry leader in antibody science and drug development • Vast experience inventing and developing therapeutic antibodies and technologies, including DARZALEX, RYBREVANT, TEPEZZA, TIVDAK & DuoBody Charles Morris, MBChB, MRCP CMO • Medical oncologist, seasoned CMO with 25+ years of global oncology drug development • Supported several approvals including including TREANDA® (bendamustine and Faslodex® (fulvestrant) • Extensive global, diversified legal and team building experience • Almost 20 years practicing law, including over 15 years in the biotech and pharmaceutical industry Amy Garabedian, MSc, JD General Counsel Steve Hurly, MSc, MBA President & CEO • 25+ years leadership experience in life sciences industry • Seasoned drug developer and biotech strategist • 20+ years of global CFO/leadership experience in biopharma • Deep expertise across investor relations, finance, capital markets, operations and information technology Fred Powell CFO ©LAVA Therapeutics 2023 5 |
| LAVA’s Proprietary Gammabody™ Platform Bispecific Gamma Delta T Cell Engagers 6 |
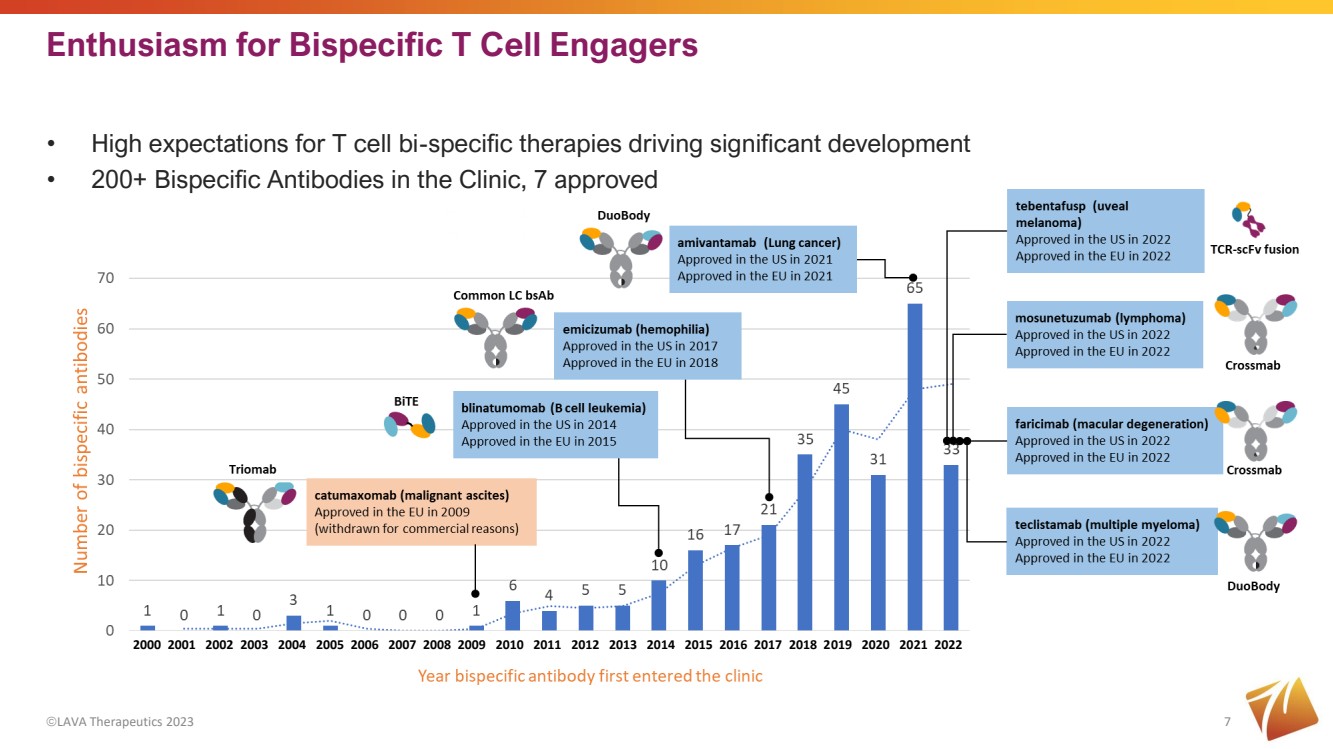
| Enthusiasm for Bispecific T Cell Engagers ©LAVA Therapeutics 2023 7 • High expectations for T cell bi-specific therapies driving significant development • 200+ Bispecific Antibodies in the Clinic, 7 approved 2000 2001 2002 2003 2004 2005 2006 2007 2008 2009 2010 2011 2012 2013 2014 2015 2016 2017 2018 2019 2020 2021 2022 |
| • High expectations for targeted T cell therapies in cancer, but often: – Narrow therapeutic window: – Cytokine Release Syndrome – On-target/off-tumor-related toxicities – Activates immunosuppressive T cells – Sporadic efficacy in solid tumors therapeutic window therapeutic effect toxic effect ©LAVA Therapeutics 2023 8 Enthusiasm for Bispecific T Cell Engagers |
| • Selecting ‘tumor-specific’ targets • Step-dosing / subcutaneous dosing • Decreasing affinity for T cells • Masking/site-specific activation • Recruiting alternative effector cells • Address only narrow target range, and/or • Cumbersome, and/or • Strongly decrease potency ©LAVA Therapeutics 2023 9 Strategies for Widening the Therapeutic Window |
| Gammabody™ Platform: Bispecific γδ T Cell Engagers DIFFERENTIAL APPROACH A versatile bispecific antibody platform for developing novel cancer therapeutics MECHANISM OF ACTION LAVA's proprietary bispecific antibodies are designed to: – Target Vγ9Vδ2 T cells to tumor antigens initiating selective tumor cell killing while sparing normal cells – Carry a low potential for on-target/off-tumor toxicity and cytokine release syndrome (CRS) ©LAVA Therapeutics 2023 10 OFF-THE-SHELF THERAPEUTICS ✓ Fully modular platform ✓ High developability ✓ Small size favors tumor penetration ✓ Proven quality of antibody products ✓ 2 formats in the clinic : bsVHH and bsVHH-Fc |
| Bispecific γδ T cell-engagers aim to harness innate & adaptive immunity • Largest γδ-T cell subset in blood: (~90-95% of total γδ-T cells) • Natural ability to recognize and kill tumor cells • Highly cytotoxic • Relatively abundant in tumor-infiltrating lymphocytes • Presence of γδ T cells associated with improved outcomes in cancer patients • Recognize tumors through phosphoantigen-BTN2A1/3A1 complex • Consistent proinflammatory cytotoxic effector T cell population • Does not contain immune-dampening regulatory T cell subsets • Ability to present antigen and orchestrate immune responses ©LAVA Therapeutics 2023 11 Innate Immunity Adaptive Immunity Adapted from Dranoff G., Nature Rev. Cancer 2004; 4: 11-22 |
| Off-the-Shelf Gammabody™ Platform: Enhances Innate Tumor Recognition by Directing Vγ9Vδ2 T Cells to the Cancer Cells LAVA’s Gammabody™ directs Vγ9Vδ2 T cells to tumors with high affinity to induce direct tumor cell killing and orchestrate an immunological cascade of anti-cancer responses and while retaining tumor selectivity Vγ9Vδ2 T cells recognize stress signals TCR interacts with pAg-butyrophilin complex Conditionally activate Vγ9Vδ2 T cells upon crosslinking with tumor associated antigen (TAA) Gammabody™ Provides Tumor Recognition to Trigger Vγ9Vδ2 T Cell-Mediated Immunity 1 2 Retains recognition of natural stress signals Natural Activation Mechanism 1 ©LAVA Therapeutics 2023 12 |
| Cascade of Anti-Cancer Responses – Potential Translation to Favorable Therapeutic Window Efficacy: • Potent killing of cancer cells (EC50s in the low picomolar range) • No co-activation of immune-suppressive Tregs which dampen antitumor efficacy of cytotoxic T cells • Orchestrate innate and adaptive immune responses, potentially resulting in potent and durable responses • Activity against hematologic malignancies and solid tumors, including immunologically “cold” tumors • Potential for expansion of V9V2 T cells can result in an increased number of anti-tumor V9V2 T cells in the tumor Safety: In addition to direct tumor cell killing, V9V2 T cells have the potential to orchestrate an immunological cascade response that includes activation of innate and adaptive immune cells in the tumor microenvironment • Conditional activation with high accuracy • Greatly reduced potential for cytokine release syndrome (CRS); No evidence of CRS in NHP studies Adapted from Dranoff G, Nature Rev Cancer 2004; 4: 11-22 Kabelitz D et al., Cell Mol Immunol 2020; 17: 925–939 © 13 LAVA Therapeutics 2023 |
| Gammabody™ can induce robust gamma delta T cell expansion and can amplify the anti- tumor immune response via downstream activation of other immune cells while avoiding co-activation of immunosuppressive T cells such as Tregs Expansion Cascade Response No Treg Activation Gammabody™ - Mediated Expansion of Vγ9Vδ2 T Cells no bsTCE CD1d Gammabody™ CD40 Gammabody™ A431 Treg EGFR-CD3 TCE EGFR Gammabody™ PBMC + tumor + tumor + EGFR Gammabody™ N=4; *: p<0.05, **: p<0.01 Data on file: LAVA Therapeutics N.V. ©LAVA Therapeutics 2023 14 Expansion & Cascade Response Without Treg Activation in Preclinical Models |
| Gammabody™ Can Selectively Kill Cancer Cells While Sparing Healthy Cells in Hematologic Malignancy and Solid Tumor models • 2:1 ratio ( T cells : Target cells) • Similar CD20 expression levels on C1R neo and B-cells CD20 Gammabody™ Mediated Killing PSMA Gammabody™ Mediated Killing Preferential killing of cancer versus healthy cells demonstrated in vitro and ex vivo; May prevent on-target/off-tumor mediated toxicity and allow for targeting of widely expressed tumor associated antigens Prostate Cancer Normal Prostate Medium + PSMA Gammabody™ + V9V2 T cells + V9V2 T cells + PSMA Gammabody™ **** p<0.001 Data on file: LAVA Therapeutics N.V. ©LAVA Therapeutics 2023 15 |
| Non-Clinical Safety Data Indicate Good Tolerability • Non-clinical safety studies using Gammabody™ molecules designed for cross-reactivity support the benign safety profile of the platform • NHP studies completed with Gammabody™ molecules targeting CD1d, CD20 and EGFR – CD1d, CD20 targeting surrogate Gammabody™ (without Fc) were dosed up to 10 mg/kg (4 hr infusion, 4 doses, every 2 days) and biweekly at 1 mg/kg for 1 month – EGFR targeting surrogate Gammabody™ (without Fc) was dosed up to 10 mg/kg (4 hr infusion, 4 doses, every 2 days) – EGFR-targeting surrogate Gammabody™ (Fc-containing) was dosed up to 23 mg/kg (0.5 hr infusion, 4 weekly doses) • No signs of cytokine release syndrome, no changes in general health parameters, relevant clinical chemistry, hematology or histopathology observed • In contrast, EGFR-targeting is severely toxic for first generation bsTCEs – NHPs infused with a CD3xEGFR BiTE required euthanasia within 3 days at doses that were 200- fold lower (on a molar basis) compared to an EGFR Gammabody with cell death observed in all tissues expressing EGFR (Lutterbuese et al., PNAS 2010) ©LAVA Therapeutics 2023 16 |
| Gammabody™ Platform: A Novel T cell engager approach for cancer therapy γδ T cell engager platform • Highly potent (kills at picomolar concentrations) • Recruits additional immune effector cells by antigen presentation and cascade response • No activation of regulatory T-cells • Tumor-cell selective, relative sparing of healthy cells expressing the target • Low risk for on-target / off tumor toxicity • Low risk for CRS anticipated • Potential for a wide therapeutic window • Applicable to hematological and solid tumor indications (including ‘cold’ tumors) ©LAVA Therapeutics 2023 17 conditional T cell activation high low medium tumor killing potency tumor selectivity regulatory T cell activation tolerability conditional T cell activation high low medium tumor killing potency tumor selectivity regulatory T cell activation tolerability LAVA’s γδ T cell engagers CD3-based T cell engagers |
| Clinical-stage company 18 |
| 19 LAVA-051 Targets CD1d to Activate Vγ9Vδ2 T Cells and iNKT Cells for the Potential Treatment of CLL, MM & AML |
| Format • Humanized bispecific single domain antibody (bsVHH) of 27kDa ‒ Short plasma half-life, prolonged functional half-life through high affinity TCR binding Mechanism of Action • Engages Vγ9Vδ2 T cells to mediate potent killing of CD1d-expressing tumor cells – Activates iNKT cells to mediate killing of CD1d-expressing tumor cells as a secondary mechanism of action – CD1d is expressed on tumor cells in CLL, MM and AML – Pre-clinical data support mechanism of action, anti-cancer activity, effector cell expansion and tumor selectivity Status • Phase 1/2a clinical trial ongoing in MM, CLL and AML LAVA-051: First-in-Class Gammabody™ Targeting CD1d 20 |
| LAVA-051: Pre-Clinical Data Support Mechanism of Action and Function 21 Expansion of Vγ9Vδ2 T and iNKT cells Lysis of patient tumor cells Adapted from Lameris et al., submitted Data on file: LAVA Therapeutics N.V ©LAVA Therapeutics 2023 0 20 40 60 80 100 CLL specific lysis (relative %) 0 20 40 60 80 100 MM 0 20 40 60 80 100 AML negative control LAVA-051 0 5 10 15 20 iNKT cells 0 20 40 60 80 Vγ9Vδ2 T cells fold expansion • LAVA-051 triggers expansion of Vγ9Vδ2 T and iNKT cells in the presence of CD1d-positive tumor cells • LAVA-051 mediates Vγ9Vδ2 T and iNKT cell-mediated cytotoxicity of patient CLL, MM and AML cells |
| LAVA-051 Phase 1/2a in Hematological Malignancies • Primary objectives: investigate safety and tolerability of LAVA-051 and determine the recommended Phase 2 dose • Secondary objectives: include evaluation of PK, PD, immunogenicity and preliminary antitumor activity • LAVA-051 administered as 2-hour infusion (IV), or subcutaneous injection (SC) (day 1, 8 and twice a week thereafter) * Cohort 5 only: 2nd dose administered SC, remaining doses IV Clinicaltrials.gov NCT04887259 ©LAVA Therapeutics 2023 22 |
| LAVA-051 – Initial Phase 1 Data - Safety • LAVA-051 has reached a dose of 200 mg (~400x the starting dose) in MM and CLL patients • Most observed AEs have not been suspected to be related • Frequency and severity of AEs have not correlated with increasing dose levels • No CRS and no ICANS (ASTCT) and no clinically relevant increase in the CRS-related cytokine IL-6 (Data cut-off date: 11 Nov 2022) ASH 2022 abstract #2014 ICANS = Immune Effector Cell Associated Neurotoxicity Syndrome; DLT = Dose Limiting Toxicity; ASTCT = American Society for Transplantation and Cellular Therapy Data on file: LAVA Therapeutics N.V ©LAVA Therapeutics 2023 |
| 24 LAVA-051 – Initial Phase 1 Data – Pharmacodynamics • Pharmacodynamic parameters reflect changes expected for the LAVA-051 mechanism of action – Vγ9Vδ2-T cell activation markers (CD25 and CD69) upregulated following dosing – Maximum Vγ9Vδ2 T cell receptor occupancy (RO) increased with dose ASCO 2022 abstract 2577; ASH 2022 abstract #2014 Data on file: LAVA Therapeutics N.V ©LAVA Therapeutics 2023 |
| 25 LAVA-051 – Pharmacodynamics ASH 2022 abstract #2014 Data on file: LAVA Therapeutics N.V ©LAVA Therapeutics 2023 Pharmacokinetics 1st dose IV, 2nd dose SC patient 32-001 cohort #5 IV SC IV • Linear LAVA-051 pharmacokinetics • SC bioavailability 74% compared to IV (based on data from Pt 32-001) |
| LAVA-051 – Initial Phase 1 Data - Patient Characteristics and Time on Treatment MM/CLL 6/6 Male/Female 8/4 Median age (range) 69 (59-76) Prior therapies, median (range) – MM/CLL 4 (3-5) / 5.5 (4-13) ASH 2022 abstract #2014, corrected Data on file: LAVA Therapeutics N.V ©LAVA Therapeutics 2023 * Death due to COVID-19 Data cut-off: 11 NOV 2022 |
| LAVA-051 – Initial Phase 1 Data - Potential Signs of Activity CLL • Patient with R/R CLL (15 mg) • Temporary enlargement and tenderness of several involved lymph nodes accompanied by grade 2 fever during Cycle 1 ‒ Resembled a tumor-flare reaction, as reported in CLL with lenalidomide • Patient assessed as having stable disease • Percent of clonal B cells in peripheral blood decreased • Numbers of CD1d expressing monocytes remained similar MM • High-risk MM patient (45 mg) • 4 prior lines of therapy within 6 years from diagnosis • Refractory to last 3 lines of treatment • 23% reduction in M-protein • Both patients ceased treatment due to COVID EHA 2002 abstract #1463 R/R = Relapsed/Refractory Permission for photo obtained Data on file: LAVA Therapeutics N.V ©LAVA Therapeutics 2023 0 25 50 75 100 125 0.01 0.1 1 Treatment days Cell count (x10 9 /L) Lymphocytes Clonal B cells Monocytes |
| LAVA-051: Summary of Initial Phase 1 Data Presented • LAVA-051 is a next-generation bispecific γδ T cell engager designed for a broad therapeutic window • LAVA-051 has reached a dose of 200 mg (400x the starting dose) in MM and CLL patients – Most observed Adverse Events (AEs) have not been suspected to be related to LAVA-051 treatment – Frequency and severity of AEs have not correlated with increasing dose levels – No Cytokine Release Syndrome (CRS) and no ICANS (ASTCT - criteria) – No significant increase in the CRS-related cytokine IL-6 • Linear pharmacokinetics and satisfactory SC bioavailability • PD parameters reflect changes as expected per Mechanism of Action • Potential signs of clinical activity • Trial continuing, including US sites (IND cleared) and evaluation of SC dosing ICANS = Immune Effector Cell Associated Neurotoxicity Syndrome; ASTCT = American Society for Transplantation and Cellular Therapy; DLT = Dose Limiting Toxicity ©LAVA Therapeutics 2023 |
| LAVA-1207 Gammabody™ that Activates Vγ9Vδ2 T Cells by Targeting PSMA for the Treatment of mCRPC |
| LAVA-1207: PSMA-targeting Gammabody™ for Prostate Cancer Format • Contains a Fc domain for extended plasma half-life; silenced to avoid off-target T cell activation • Small size (compared to regular IgG antibodies) to facilitate tumor penetration Mechanism of Action • Specifically directs Vγ9Vδ2 T cells to PSMA-expressing tumor cells – PSMA is a well-validated tumor target • Mediates potent killing of PSMA-positive tumor cells • Pre-clinical data support mechanism of action, anti-cancer activity & selectivity Status • Phase 1/2a trial in mCRPC; patient recruitment ongoing (NCT05369000) Data on file: LAVA Therapeutics N.V ©LAVA Therapeutics 2023 30 10 -3 10 -2 10 -1 10 0 10 1 10 2 10 3 10 4 0 50 100 150 concentration LAVA-1207 (pM) % specific killing PC-3 (PSMA-neg) LNCaP (PSMA-high) LNCaP-PSMA KO |
| LAVA-1207: Preclinical Data Support Activity and Selectivity in Patient Samples • LAVA-1207 triggers activation of autologous Vγ9Vδ2 T cells in the presence of patient-derived tumor cells • LAVA-1207 induces selective tumor cell lysis Data on file: LAVA Therapeutics N.V ©LAVA Therapeutics 2023 31 Medium + LAVA-1207 0 5 10 15 20 CD107a expression (%) Tumor-infiltrating Vγ9Vδ2 T cells Medium + LAVA-1207 0 20 40 60 80 CD107a expression (%) PBMC Vγ9Vδ2 T cells Control + PBMC + LAVA-1207 + PBMC + LAVA-1207 0 25 50 Tumor tissue Lysed cells (% relative to control) Control + PBMC + LAVA-1207 + PBMC + LAVA-1207 0 25 50 Normal tissue Lysed cells (% relative to control) Vγ9Vδ2 T cell degranulation Preferential lysis of prostate tumor cells |
| LAVA-1207 – Phase1/2a Study Design 32 • Dose escalation in patients with mCRPC (EudraCT 2021-001789-39; NCT05369000) • Primary objectives: investigate safety and tolerability of LAVA-1207 • Secondary objectives: evaluate PK, PD, immunogenicity and preliminary signs of antitumor activity • LAVA-1207 administered via IV infusion every 2 weeks ASCO GU 2023 abstract #153 Data on file: LAVA Therapeutics N.V ©LAVA Therapeutics 2023 |
| LAVA-1207 – Patient Baseline Characteristics 33 N=20 Age, median (range) 68 (51-76) Years since diagnosis, median (range) 9 (3-21) Prior systemic therapies, median (range) 4 (3-10) Location of metastases Bone 19 Lymph node 14 Lung 2 Liver 5 Other visceral 2 Type of progression PSA 17 Bone 12 Nodal 12 Visceral 10 ASCO GU 2023 abstract #153 Data on file: LAVA Therapeutics N.V ©LAVA Therapeutics 2023 |
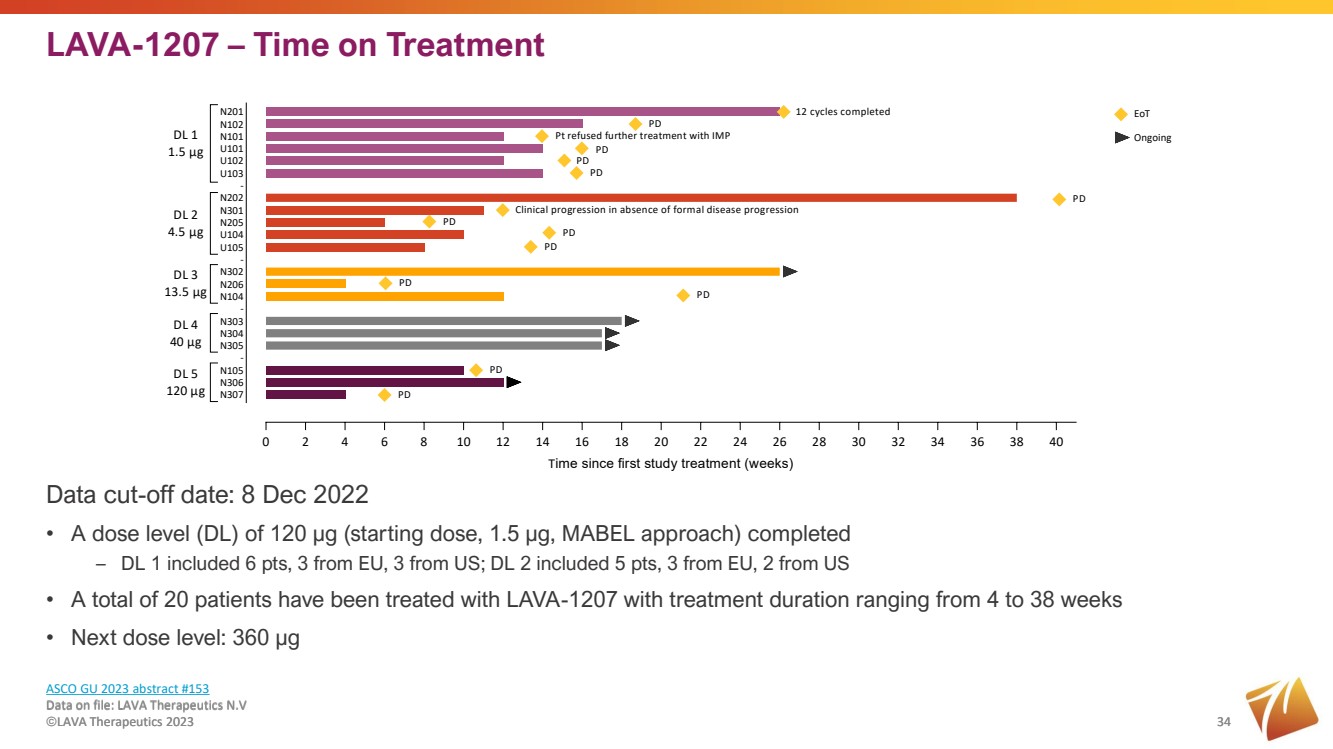
| LAVA-1207 – Time on Treatment Data on file: LAVA Therapeutics N.V ©LAVA Therapeutics 2023 34 Data cut-off date: 8 Dec 2022 • A dose level (DL) of 120 µg (starting dose, 1.5 µg, MABEL approach) completed – DL 1 included 6 pts, 3 from EU, 3 from US; DL 2 included 5 pts, 3 from EU, 2 from US • A total of 20 patients have been treated with LAVA-1207 with treatment duration ranging from 4 to 38 weeks • Next dose level: 360 µg 34 0 2 4 6 8 10 12 14 16 18 20 22 24 26 28 30 32 34 36 38 40 N307 N306 N105 - N305 N304 N303 - N104 N206 N302 - U105 U104 N205 N301 N202 - U103 U102 U101 N101 N102 N201 Time since first study treatment (weeks) DL 1 1.5 μg DL 2 4.5 μg DL 3 13.5 μg DL 4 40 μg DL 5 120 μg Pt refused further treatment with IMP PD PD 12 cycles completed PD PD PD PD PD PD PD PD Clinical progression in absence of formal disease progression EoT Ongoing PD PD ASCO GU 2023 abstract #153 Data on file: LAVA Therapeutics N.V ©LAVA Therapeutics 2023 |
| LAVA-1207 – Initial Phase 1 Data - Safety 35 Data cut-off date: 8 Dec 2022 • Most observed AEs not suspected to be related and no DLT • Treatment emergent AEs (TEAEs) that were suspected to be related were grade 1 or 2 • No increase in severity or frequency of TEAEs with increasing doses and no patient discontinued treatment due to AE • One grade 4 AE occurred (spinal cord compression, DL 5), which was non-related 0 20 40 60 80 100 Fatigue Anemia Back pain Pain AST increase Alkaline phosphatase increase Vomiting Nausea Anorexia Fall Tumor pain Hematuria Tingling Infusion related reaction Cytokine release syndrome Fever Flu-like symptoms Diarrhea Constipation Dry mouth Dyspepsia Dyspnea Edema Chills Hot flashes Hypertension Hypocalcemia Lymphocyte count decreased LDH increase Lung embolism Urinary tract obstruction Urinary tract infection 100 80 Frequency (%) All TEAE >5%, by grade TEAE suspected to be related >5%, by grade Grade 1 Grade 2 Grade 3 N=20 60 40 20 DL1 - N=6 (1.5 μg) DL2 - N=5 (4.5 μg) DL3 - N=3 (13.5 μg) DL4 - N=3 (40 μg) DL5 - N=3 (120 μg) 0 Occurrence of IRR and CRS per dose level no IRR or CRS IRR CRS 2 2 1 2 1 Numbers above symbols indicate grade Prophylactic medication As of 20 Oct 2022, prophylaxis with antipyretic and antihistaminic treatment to mitigate potential fever, IRR or CRS was implemented. ASCO GU 2023 abstract #153 Data on file: LAVA Therapeutics N.V ©LAVA Therapeutics 2023 |
| LAVA-1207 – Pharmacokinetics Data cut-off date: 8 Dec 2022 • Pharmacokinetics of LAVA-1207 appears linear 36 0 24 48 72 96 120 144 168 1,000 10,000 Dose level 4 Time since infusion start (h) LAVA-1207 (ng/L) 5000 N303 N304 N305 0 24 48 72 96 120 144 168 1,000 10,000 Dose level 5 Time since infusion start (h) LAVA-1207 (ng/L) 30,000 N105 N306 N307 0 500,000 1,000,000 1,500,000 2,000,000 120 Dose vs. AUC for Cycle 1 Dose (μg) AUC(0-∞) (h*pg/mL) Subject ID N202 N301 N104 N206 N302 N303 N304 N305 N105 N306 N307 4.5 13.5 40 DL 2 DL 3 DL 4 DL 5 ASCO GU 2023 abstract #153 Data on file: LAVA Therapeutics N.V ©LAVA Therapeutics 2023 |
| LAVA-1207 – Pharmacodynamics Data cut-off date: 8 Dec 2022 • Pharmacodynamics reflect changes expected as per MoA • Pronounced drop in Vγ9Vδ2-T cell frequency 2 hr after dosing, suggesting Vγ9Vδ2-T cell re-distribution, with subsequent recovery • Vγ9Vδ2-T cell activation markers (CD25 and CD69) upregulated following dosing • Receptor occupancy (RO) was detectable up to day 14 after EoI, with peak levels ranging from 6.1% to 12.6% 37 0 2 4 6 8 10 0 50 100 150 25 50 Vγ9Vδ2 T cells - % of CD45 Change compared to predose Time since first study treatment (days) Relative change (%) 0 2 4 6 8 10 0 10 20 30 40 25 50 Vγ9Vδ2 T cells - % CD25+ Time since first study treatment (days) %CD25+ 0 2 4 6 8 10 0 20 40 60 80 100 25 50 Vγ9Vδ2 T cells - % CD69+ Time since first study treatment (days) %CD69+ 0 2 4 6 8 10 0 10 20 25 50 Vγ9Vδ2 T cells - % LAVA-1207 receptor occupancy (RO) Treatment day %RO N304 N305 N303 dosing ASCO GU 2023 abstract #153 Data on file: LAVA Therapeutics N.V ©LAVA Therapeutics 2023 Dose level 4 – 40 µg |
| LAVA-1207 – Preliminary Signs of Antitumor Activity 38 • Out of 14 iRECIST evaluable patients, 8 had iSD at week 8. 38 Data cut-off date: 8 Dec 2022 Patient N304 – 40 mg • Largest overall decrease in PSA was 61% (46% vs baseline) • Per treating physician, the patient improved clinically with improvement in pain and fatigue • Ongoing in the study 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 -50 0 50 100 Best PSA response PSA change from C1D1 (%) 220 198 191 147 133 129 120 0 2 4 6 8 10 12 14 0 200 400 600 800 PSA levels - Pt N304 Cohort 4 Time since first study treatment (weeks) PSA (μg/L) ASCO GU 2023 abstract #153 Data on file: LAVA Therapeutics N.V ©LAVA Therapeutics 2023 |
| Summary of Initial Phase 1 Data Presented • LAVA-1207 is a PSMA targeting bispecific antibody belonging to a novel class of γδ T cell engagers (Gammabody™) • LAVA-1207 has reached a dose of 120 mg (starting dose 1.5 mg) without the occurrence of high-grade (>2) CRS or DLTs in therapy refractory mCRPC patients – Frequency and severity of AEs do not appear to be dose-dependent – Most observed AEs were not suspected to be related – Next dose level (360 mg) is ongoing • Preliminary signs of clinical activity observed with disease stabilization and PSA reduction during dose escalation • Pharmacodynamics reflect changes as expected per MoA • Dose escalation continues in both the EU and the US 39 ASCO GU 2023 abstract #153 Data on file: LAVA Therapeutics N.V ©LAVA Therapeutics 2023 |
| 40 LAVA-1223 – Licensed to Seagen Gammabody™ for the treatment of EGFR-expressing solid tumors |
| LAVA-1223: EGFR-Targeting Gammabody™ Format • Gammabody™ format containing a silenced Fc domain Mechanism of Action • Induces preferential lysis of EGFR-expressing tumor cells while relatively sparing EGFR-expressing normal cells Status • Exclusive worldwide license agreement with Seagen Inc. • Seagen to develop and commercialize LAVA-1223, potential for milestones of up to approximately $650 million and royalties 41 King et al., submitted Data on file: LAVA Therapeutics N.V ©LAVA Therapeutics 2023 – Licensed to Seagen Patient derived tumor tissue Patient derived non-tumor tissue |
| 42 CD123 Targeting Gammabody™ for the Treatment of Hematologic Malignancies LAVA-1266 |
| Mechanism of Action • Induces preferential lysis of CD123-expressing tumor cells while relatively sparing CD123-expressing normal cells – CD123 is overexpressed in a wide range of hematological malignancies Status • CTA/IND enabling studies ongoing; filing anticipated in 2024 LAVA-1266: CD123-Targeting Gammabody™ In Development for Treating Hematological Malignancies Data on file: LAVA Therapeutics N.V ©LAVA Therapeutics 2023 0.01 0.1 1 10 100 1000 0 20 40 60 80 100 LAVA-1266 concentration [pM] Specific cell death (%) EC50 ~ 8 pM Potent lysis of primary AML cells 0.01 0.1 1 10 100 1000 0 20 40 60 80 100 LAVA-1266 concentration (pM) Specific cell death (%) CD123+ tumor cell line CD123+ healthy cells (donor 1) CD123+ healthy cells (donor 2) Preferential lysis of tumor cells |
| Milestones 44 |
| MM: multiple myeloma CLL: chronic lymphocytic leukemia AML: acute myeloid leukemia PSMA: prostate-specific membrane antigen EGFR: epidermal growth factor receptor mCRPC: metastatic castration-resistant prostate cancer Gammabody™ Pipeline: Potential in Hematological and Solid Tumor Indications 45 Candidate Target Indication(s) Discovery Preclinical Phase 1 Phase 2 Milestones LAVA-051 CD1d MM CLL AML • Initial data released ASCO 2022 • Additional data in 2023 LAVA-1207 PSMA mCRPC • Initial data released ASCO GU 2023 • Additional data 2H 2023 LAVA-1223 EGFR Solid Tumors • Licensed to Seagen Sep 2022 LAVA-1266 CD123 Hematologic Malignancies • IND/CTA filing expected in 2024 LAVA-1278 CD40 Hematologic Malignancies Janssen Collaboration undisclosed Hematologic malignancy Solid Tumor ©LAVA Therapeutics 2023 |
| Gamma delta T cell engagers for the development of next-generation cancer therapeutics Corporate Presentation February 2023 |